Enable Discovery and Validation of New Therapeutics with Curi Bio's
High Fidelity DMD Tissue Model
Duchenne muscular dystrophy (DMD) is a genetic disorder characterized by progressive muscle degeneration and weakness due to the alterations of a protein called dystrophin that helps keep muscle cells intact1. A number of mutations within the dystrophin gene can result in DMD or a less severe disorder known as Becker Muscular dystrophy. The earliest clinical symptom of DMD is primarily skeletal muscle weakness, followed by progressive loss of cardiac and diaphragm function.
1MDA.org
2D Morphology
Myoblasts display typical compact morphology with doubling times between 20-24 hours. Myoblast purity is above 80% as revealed through Desmin staining. Within just five days, the myoblasts rapidly fuse at high density, forming striated, multinucleated, and contractile myotubes.
3D Morphology
3D morphology is stable through 43 days of culture. Increased density and reduced size of the 3D constucts indicates greater compaction as the tissues remodel and mature over time.
DMD Tissues Show Significant Contractile Deficits and Greater Fatigue Compared to Isogenic Control Engineered Muscle Tissues (EMTs)
Skeletal EMTs bearing a dystrophin-null phenotype (a model of DMD) exhibited significant reductions in contractile twitch and tetanic force and kinetics compared with healthy (wild type) isogenic controls as measured on the Mantarray platform, . A significant disease phenotype presents early in this model and persists through over a month in 3D culture. Delayed contraction and relaxation rates indicate impaired muscle function in the DMD line.
DMD tissues also display greater fatigue following repetitive contractions by electrical stimulation, similar to in vivo models of the disease and clinical manifestation. This shows the potential to utilize this model to precisely measure a drug's affect on fatiguability.
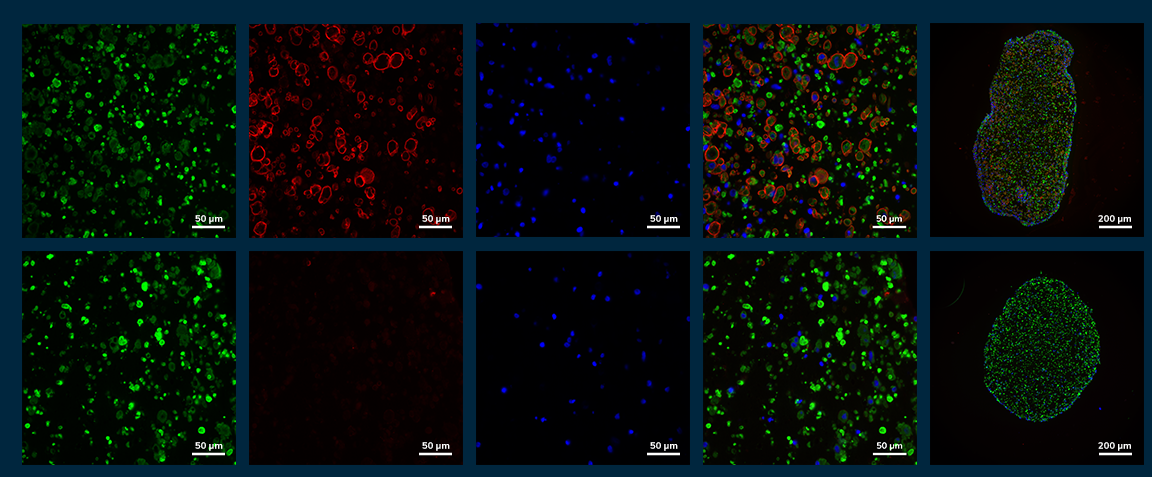
Histological analysis of tissue cross sections showed distribution of myosin positive myotubes throughout the EMT with a pronounced dystrophin ring stained at the cell membrane in healthy tissues. DMD tissues, as expected, completely lacked dystrophin rings surrounding myosin positive myotubes. These data demonstrate the ability of Mantarray to uniquely stratify healthy and diseased phenotypes to recapitulate native developmental architecture and muscle function.
Positive Force-Frequency Relationship in iPSC-DMD Model Closely Mimics That Seen in Human Skeletal Muscles
The force-frequency relationship, recorded on Curi Bio’s Mantarray platform, presents the amount of contractile force skeletal muscle produces as a function of electrical stimulation frequency of activation. MantaReady isogenic cells not only show a clear disease phenotype, but also demonstrate the ability of this model to recapitulate in vivo muscle function to improve the translatability of pre-clinical testing.
Learn More About MantaReady™
Curi Bio’s MantaReady suite of products adds efficiency to your 3D muscle tissue experiments.
If you are interested in learning more about how MantaReady products can accelerate your research, please contact us to arrange a conversation with our team of experts.