Making the Inaccessible Accessible
Monogenic diseases often have a spectrum of genetic mutations and factors. Symptoms, timelines, and treatments rarely follow the same script. With rare and ultra-rare disorders, each patient is unique, and therapies may have to be tailored to the individual. Curi Bio is accelerating the path to investigational new drugs, making personalized medicine a reality and developing potency/release assays featuring human clinically-translatable disease models.
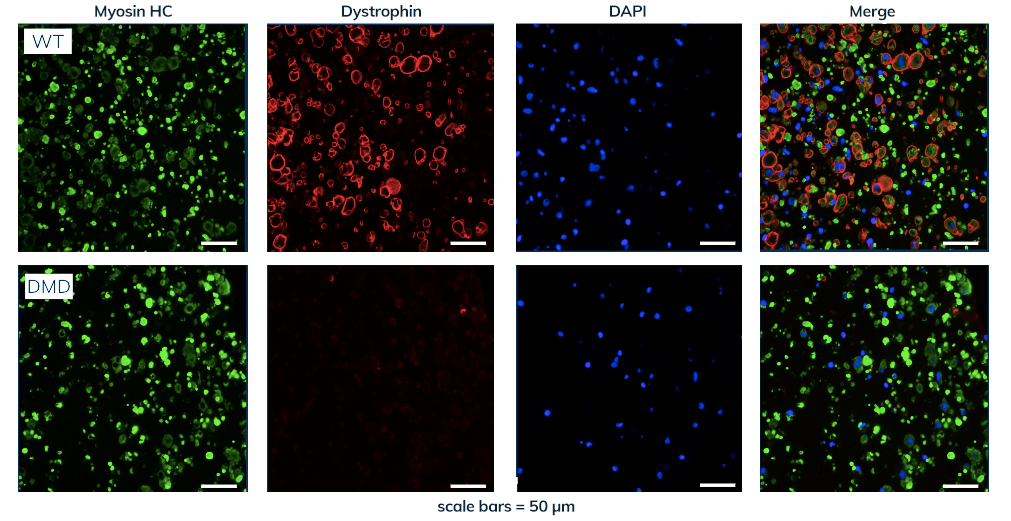
Curi Bio has licensed and/or developed advanced human tissue models for a number of monogenic disorders, including Duchenne Muscular Dystrophy (DMD) (see our case study below). Additional models include:
Myotonic Dystrophy Type 1 (DM1)
Facioscapulohumeral Muscular Dystrophy (FSHD)
X-linked Myotubular Myopathy (XLMTM)
Hypertrophic & Dilated Cardiomyopathies (HCM/DCM)
and More
Contact our team to discover how Curi Bio can help accelerate your disease research and therapeutic discoveries by providing access to our pre-made disease model library, tissue models, and tissue-specific biosystems. Curi Bio is actively pursuing additional partnerships to create new disease models.