Curi Bio Launches Mantarray™ Platform – 3D Engineered Muscle Tissues for Discovery of New Therapeutics
Accelerating Drug Discovery with Human-Relevant 3D Engineered Muscle Tissue Analysis
Originally posted on BUSINESS WIRE—SEATTLE, WA—Curi Bio, a leading developer of human stem cell-based platforms for drug discovery, today announced the commercial launch of the Mantarray™ platform for human-relevant 3D heart and skeletal engineered muscle tissue (EMT) contractility analysis. Curi’s Mantarray platform enables the discovery of new therapeutics by providing parallel analysis of 3D EMTs with adult human-like functional profiles of healthy and disease models. Over the past three years, Curi Bio has developed the Mantarray platform in close beta testing and development partnerships with several leading global pharma companies, biotech companies, and regulatory agencies for use in their drug discovery and development projects. With this commercial launch, the Mantarray instrument and consumables will be available worldwide, on a first-come, first-served basis.
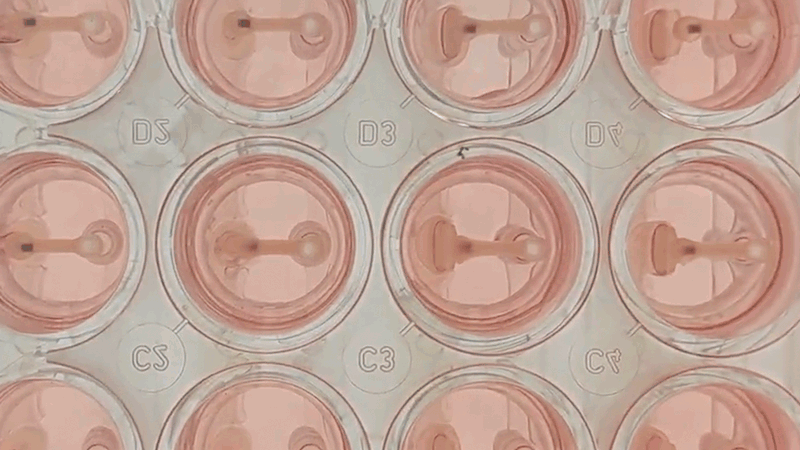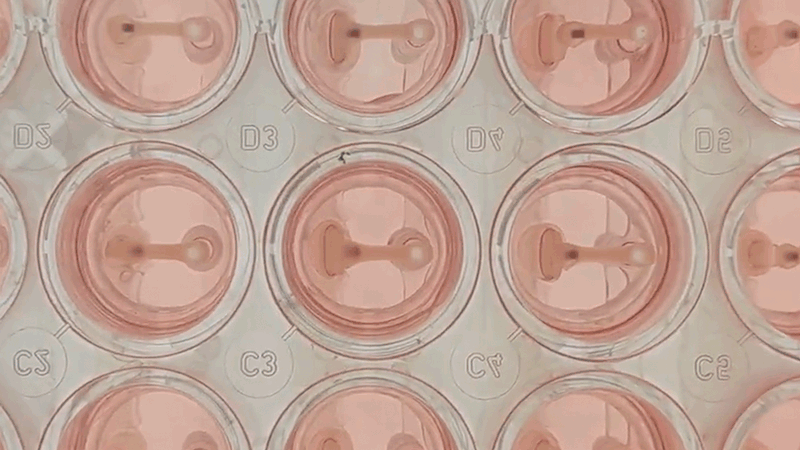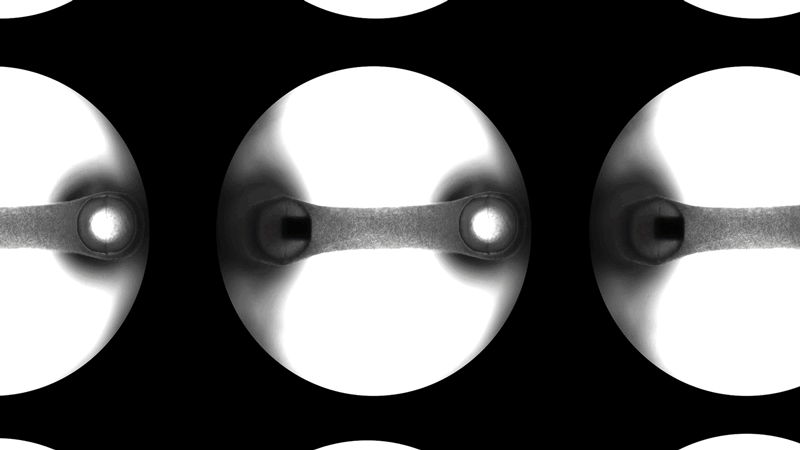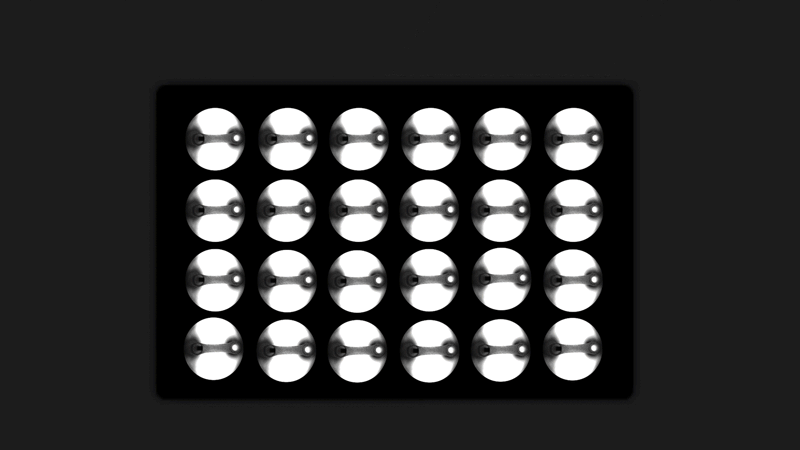
Curi Bio’s Mantarray instrument and consumable.
Lack of clinical translatability in current preclinical models, which often rely upon surrogate biomarkers or poorly translational animal models, may be to blame for the unacceptably high clinical trial failure rates plaguing the industry. For cardiac and skeletal muscle diseases, direct assessment of contractile output constitutes the most reliable metric to assess overall tissue function, as other ‘proxy’ measurements are poor predictors of muscle strength. 3D EMTs derived from human induced pluripotent stem cells (iPSCs) offer a promising route to model the contractile deficiencies seen in the heart and muscles of patients. However, the bioengineering strategies required to generate these predictive models at sufficient scale are oftentimes out of reach for most researchers.
Curi’s solution to this problem is the Mantarray platform: an easy-to-use, scalable, and flexible biosystem for assaying EMTs. The Mantarray platform leverages a proprietary, label-free, electromagnetic measurement system for parallel contractility assessment of up to 24 3D EMTs simultaneously and can utilize a variety of contractile cell types. The system has built-in electrical stimulation capabilities, enabling users to easily create individual stimulation protocols for each well, for both short and long-term pacing of cardiac and skeletal muscle models. Curi has developed consumable Mantarray plates that are specifically designed for cardiac and skeletal muscle applications. 3D Mantarray tissue models will also be compatible with Curi’s new Nautilus system (optical mapping system for voltage and calcium analysis).
Additionally, Curi offers services and partnerships leveraging the Mantarray technology for applications in drug discovery, disease modeling, and safety and efficacy screening. Companies interested in fully accessing or licensing Curi’s proprietary disease models, next-generation iPSC platforms, gene therapy technologies and technical expertise can do so with Curi’s new Technology Access Partnership program. Further details of this Technology Access Partnership program will be announced at a later date.
High-fidelity models of human diseases can be created and tested on the Mantarray platform using human iPSC-derived cells or patient-derived myoblasts from healthy or affected individuals. For example, Mantarray 3D EMTs can be formed from cells harboring disease-causing mutations (patient-derived or gene-edited; iPSC-derived or primary) to model human diseases such as Duchenne muscular dystrophy, myotonic dystrophy type 1, hypertrophic and dilated cardiomyopathies, and more. Multi-modal data captured with the 3D platform shows enhanced disease stratification for a number of monogenic disorders. Curi Bio is accelerating the path to investigational new drugs, making personalized medicine a reality through clinically-translatable human disease models.
Safety and efficacy testing can also be performed with the Mantarray’s novel magnetic detection of drug-induced contractile changes. Acute drug responses can be measured on the order of seconds to minutes with enough sensitivity to detect dose-response-like behavior. Alternatively, chronic experiments can be performed over the course of days to weeks for longer-term studies. Mantarray brings clinically-relevant functional data into the earliest stages of preclinical testing of new therapeutics.
“At Curi Bio, our goal is to provide researchers with innovative solutions to accelerate the discovery of new medicines,” said Curi CEO Michael Cho. “The Mantarray platform provides drug developers with clinically-relevant preclinical models that more closely recapitulate human cardiac and skeletal muscle tissues. With this key milestone, Curi is closing the gap between preclinical results and clinical impact. Our clients are using this data in direct support of IND applications.”
To learn more about how the Mantarray system can improve the predictive power of your experiments, or about Curi’s other human-relevant preclinical platform technologies and services, please reach out at www.curibio.com/contact.
About Curi Bio
Curi Bio’s preclinical discovery platform combines human stem cells, systems, and data to accelerate the discovery of new medicines. The Curi Engine is a comprehensive, bioengineered platform that integrates human iPSC-derived cell models, tissue-specific biosystems, and AI/ML-enabled phenotypic screening data. Curi’s suite of human stem cell-based products and services enable scientists to build more mature and predictive human iPSC-derived tissues—with a focus on cardiac, musculoskeletal, and neuromuscular models—for the discovery, safety testing, and efficacy testing of new drugs in development. By offering drug developers an integrated preclinical platform comprising highly predictive human stem cell models to generate clinically-relevant data, Curi is closing the gap between preclinical data and human results, accelerating the discovery and development of safer, more effective medicines.
For more information, please visit www.curibio.com.
Contact
Heejoon Choi
Director of Sales & Marketing, Curi Bio